38 fda health claims on food labels
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and... Label Claims for Conventional Foods and Dietary Supplements | FDA 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)

Fda health claims on food labels
Making Health Claims: Follow the Science, Not the Hype 02.06.2022 · “The FDA gets blamed for being the gatekeeper, but it’s really the science that dictates when a claim can be made. It’s hard, scientifically, to demonstrate the health benefit of a food.” Rules regarding labeling. The FDA does not just approve health claims; it also dictates how those claims can be stated on labels. For example ... Authorized Health Claims That Meet the Significant Scientific Agreement ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Qualified Health Claims | FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Fda health claims on food labels. PDF "GMO-Free" Claims and False and Misleading Food Labels—Why Is FDA AWOL? articles of food, or articles which enter into the composition of food, the package or label of which shall bear any statement, design, or device regarding such article, or the ingredients or substances contained therein which shall be false or misleading in any particular… --Pure Food and Drug Act of 1906 (P.L. 59-384, 34 Stat. 768). 12 FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Abstract The U.S. Food and Drug Administration's regulatory authority over health claims was clarified in 1990 legislation known as the Nutrition Labeling and Education Act (NLEA). CFR - Code of Federal Regulations Title 21 29.03.2022 · § 101.71 - Health claims: claims not authorized. § 101.72 - Health claims: calcium, vitamin D, and osteoporosis. § 101.73 - Health claims: dietary lipids and cancer. § 101.74 - Health claims: sodium and hypertension. § 101.75 - Health claims: dietary saturated fat and cholesterol and risk of coronary heart disease. Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified.". Authorized Health Claims: Claims that have significant scientific agreement (SSA).
Nutrient Content Claims | FDA Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ... Nutrient Content Claim vs Health Claim - LabelCalc Nutrient content claims, which are commonly used on food labels, either refer to the amount of a nutrient in a product or compare the levels of a nutrient in that food to a similar reference food. ... According to the FDA, health claims refer to the relationship between a specific food product or ingredient and a reduced risk of disease or a ... Label Claims for Food & Dietary Supplements | FDA 07.03.2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Subpart D - Specific Requirements for Nutrient Content Claims. Sec. 101.54 Nutrient content claims for "good source," "high," "more," and "high potency." (a) General requirements.
Food Label Claims: What You Can and Can't Trust - WebMD The FDA is updating its definition for this claim. Until then, companies can make the "healthy" claim if the fats in their foods are mostly mono- and polyunsaturated fats. The healthy claim also... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ... Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if... 5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease."
eCFR :: 21 CFR Part 101 -- Food Labeling § 101.1 Principal display panel of package form food. The term principal display panel as it applies to food in package form and as used in this part, means the part of a label that is most likely to be displayed, presented, shown, or examined under customary conditions of display for retail sale. The principal display panel shall be large enough to accommodate all the mandatory label ...
The Basics of Food Product Health Claims - LabelCalc For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C. Claim #3: Qualified Health Claims. Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim.
› food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
What are some examples of an FDA health claim on a food label? The FDA regulates two types of claims that food companies put on food labels: Nutrient claims and health claims. A structure/function claim describes the role of a substance intended to maintain the structure or function of the body. Structure/function claims do not require preapproval by FDA. 21 CFR 101.14 (a) (1) and (c), and 21 CFR 101.93 (f)
Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
Qualified Health Claims | FDA Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions.
Authorized Health Claims That Meet the Significant Scientific Agreement ... Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food...
Making Health Claims: Follow the Science, Not the Hype 02.06.2022 · “The FDA gets blamed for being the gatekeeper, but it’s really the science that dictates when a claim can be made. It’s hard, scientifically, to demonstrate the health benefit of a food.” Rules regarding labeling. The FDA does not just approve health claims; it also dictates how those claims can be stated on labels. For example ...


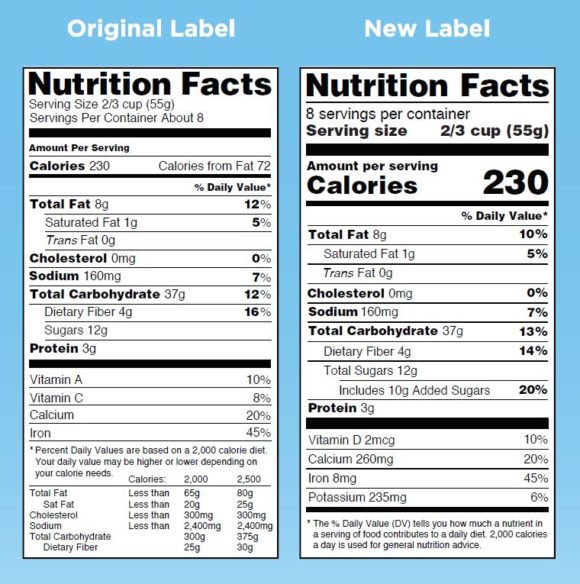








Post a Comment for "38 fda health claims on food labels"